Abstract
Introduction The extended use of peripherally inserted central catheters (PICCs) in cancer patients have simplified the management of the treatment, but also represent an important risk factor of upper limbs thrombosis in a population specially predisposed to thrombotic events. Given the clinical significance of PICC-related venous thrombosis (VT) in cancer patients, a predictive risk model could be helpful.
Methods Retrospective unicentric study in which patients with single-lumen PICCs, placed by using ultrasound guidance by a trained nursing team at our centre from January 2015 to May 2019 were included. Inclusion criteria were age ≥18 years-old, histologically confirmed diagnosis of cancer and treatment with ambulatory chemotherapy regimen. The presence of symptomatic PICC-related VT confirmed by ultrasounds, variables regarding PICC (placement, use and removal) and the main clinical and biological characteristics of the patients were recorded. For further analysis, sites of cancer were categorized into "very high risk", those with VT rates of 2-fold or higher than the median risk of the group (lung, testicular, bladder), "high risk", those with rates higher than average (gastric, pancreatic, breast, head and neck), and low risk, those with rates at or below average (colorectal, prostate, lymphoma, gynecologic, and others). The predictive accuracy of the model was assessed: discrimination was evaluated using the c-statistic representing the AUC (area under the receiver-operating characteristic), the Hosmer-Lemeshow test was used to assess model calibration and a bootstrap internal validation procedure with 1000 bootstrap resamples was performed.
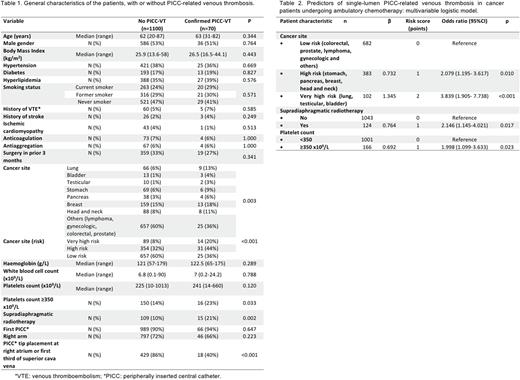
Results Seventy (6%) of 1170 patients who received PICCs, with a median time of use of 108.5 days [2-525], developed symptomatic, image-confirmed PICC-related VT. Moreover, 10 of the 70 had associated pulmonary embolism. The main characteristics of the patients with and without PICC-VT are summarized in table 1. The median time between PICC placement and thrombosis was 25.5 [1-458] days and VT was more probable on the first 100 days after placement (p=0.002).
In univariable analyses, the following variables were significantly associated with the development of symptomatic PICC-VT: primary site of cancer, pre-chemotherapy platelet count ≥350 x109/L and supradiaphragmatic radiotherapy concomitant to PICC use. In addition, PICC tip placement next to the right atrium (in the right atrium or at the lower third of the superior vena cava) is associated with a lower incidence of VT. PICC tip position only could be checked in 447 patients by chest radiography.
In the final multivariable logistic regression model (table 2), three variables emerged as risk factors independently associated with PICC-VT: primary site of cancer (very high risk or high risk), pre-chemotherapy platelet count ≥350 x109/L and supradiaphragmatic radiotherapy concomitant to PICC use. PICC tip placement was not considered in the multivariable analysis, since it is a correctable variable. To create the risk score, we assigned points to each of the three variables based on their regression coefficients (table 2). Patients were assigned to one of three risk groups based on cumulative total points: low (score 0), intermediate (score 1), and high (score ≥2). Observed PICC-related VT rates were 3.1% for the low-risk group, 6.4% for the intermediate-risk group and 12.4% for the high-risk group. In a logistic regression model with risk group as a categorical predictor, the risk classification rule was significantly associated with PICC-VT (p <0.001), with odds ratios of 2.136 (95% CI, 1.137-4.011) and 4.456 (95% CI, 2.387-8.320) for intermediate-risk and high-risk groups vs. low-risk group, respectively. C statistic was 0.662 (95%CI 0.598-0.727).
Conclusions Primary site of cancer, pre-chemotherapy platelet count and supradiaphragmatic radiotherapy concomitant to the PICC use are independent risk factors for single-lumen PICC-related venous thrombosis in cancer patients undergoing ambulatory chemotherapy. The score proposed herein could be a helpful tool to predict the risk of PICC-related VT. PICC tip placement proximal to the right atrium could reduce the risk of VT.
Disclosures
Sancho:Eli Lilly & Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biomedicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Kern Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees. Navarro:BluePrint Medicines: Consultancy; GILEAD: Research Funding; EUSA Pharma: Honoraria, Research Funding; Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.